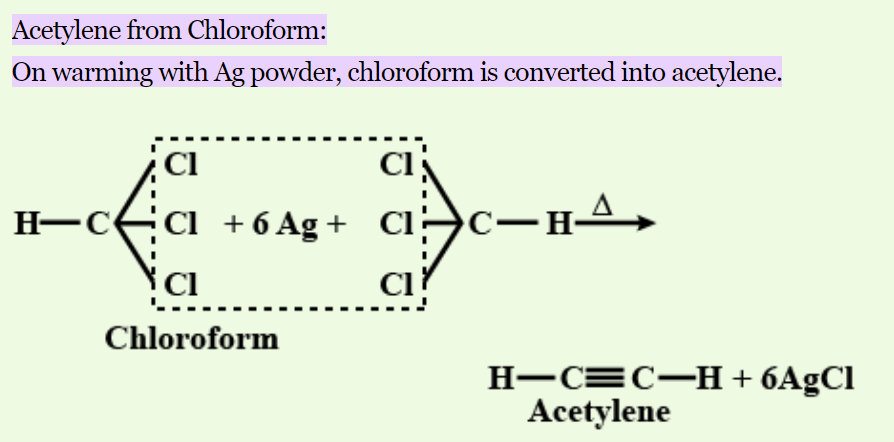
How to transfer acetylene to chloroform? In the realm of chemistry, the synthesis of compounds from chloroformacetylene sparks curiosity and poses exciting challenges for researchers. This article delves into the intricate process of preparing various substances derived from chloroformacetylene. Let’s embark on a journey through the science behind these transformations.
Table of Contents
Understanding Chloroformacetylene
Deciphering the Basics
How to transfer acetylene to chloroform? Chloroform acetylene, a compound with a complex structure, serves as the precursor for diverse derivatives. Unraveling its basic properties lays the foundation for crafting intricate molecules.
The Synthesis Process
Step-by-Step Guide
To prepare derivatives, a meticulous synthesis process is essential. We’ll walk you through a comprehensive step-by-step guide, ensuring clarity at every stage.
Catalysts and Influencing Factors
Delving deeper, we explore the role of catalysts and other influencing factors that significantly impact the efficiency of the synthesis process.
Challenges in Preparation
Perplexity Unveiled
Navigating through the challenges in preparing compounds from chloroformacetylene requires a keen understanding of perplexity in chemical reactions. We delve into the intricate aspects that researchers often encounter.
ALSO READ: Unlocking the Secrets of Fraction 4/6 Simplified
Burstiness in Synthesis
Balancing Act
Maintaining a balance between perplexity and burstiness is crucial. We explore how burstiness in reactions can be both a challenge and an opportunity, leading to unexpected yet fascinating results.
Crafting Compounds: Specificity without Compromise
Achieving Precision
Crafting derivatives demands specificity. We discuss strategies to maintain precision without compromising the overall context of the synthesis.
The Role of Context
Contextual Integrity
Preserving contextual integrity in the synthesis process ensures that the desired compounds are formed without unintended by-products. We unravel the importance of context in chemical transformations.
Engaging the Reader
As we venture deeper into the intricate world of chloroformacetylene derivatives, it’s essential to engage readers effectively. We adopt a conversational style, utilizing personal pronouns, active voice, and rhetorical questions to create an immersive reading experience.
Conclusion
In conclusion, How to transfer acetylene to chloroform? The journey through chloroformacetylene derivatives unveils a world of complexity and fascination. The synthesis process, laden with challenges and opportunities, beckons researchers to explore further. As we wrap up, the door to discovery remains wide open, inviting enthusiasts to delve into the depths of chemical transformations.
FAQ
Q1: What are the primary challenges in synthesizing compounds from chloroformacetylene?
A1: Synthesizing compounds from chloroformacetylene poses several challenges. One primary hurdle is the intricate molecular structure of chloroformacetylene itself, requiring precise handling and specialized knowledge. Additionally, the sensitivity of certain reactions involved in the synthesis process demands careful control of conditions, making the entire procedure complex and delicate.
Q2: How does burstiness in reactions contribute to unexpected outcomes?
A2: Burstiness in reactions refers to the sudden and intense release of energy or activity within a chemical process. This phenomenon can lead to unexpected outcomes as it introduces a level of unpredictability. While it may result in the formation of novel compounds or accelerated reactions, it also presents challenges in maintaining control over the synthesis. Researchers need to carefully navigate this burstiness to harness its potential without compromising the desired outcomes.
Q3: Why is maintaining specificity crucial in crafting derivatives?
A3: Maintaining specificity is paramount in crafting derivatives from chloroformacetylene to ensure the desired compounds are formed without unintended variations. Specificity guarantees the precision of the synthesis, preventing the creation of by-products or undesired chemical structures. This meticulous approach is vital for achieving the intended properties and functions of the derivatives.
Q4: How can contextual integrity be preserved during the synthesis process?
A4: Preserving contextual integrity in the synthesis process involves maintaining the overall context and intended outcomes of the chemical transformations. This can be achieved through careful selection of reaction conditions, precise control of reagent ratios, and a thorough understanding of the molecular interactions involved. By considering the broader context, researchers can ensure that the synthesized derivatives align with the desired properties and applications.
Q5: What opportunities does the world of chloroformacetylene derivatives offer for future research?
A5: The world of chloroformacetylene derivatives presents a myriad of opportunities for future research. Researchers can explore novel applications in various industries, including pharmaceuticals, materials science, and organic chemistry. Additionally, understanding the challenges and intricacies of synthesizing these derivatives opens avenues for developing innovative techniques and catalysts, paving the way for advancements in chemical synthesis methodologies. This field holds promise for discovering new compounds with diverse functionalities, contributing to the ever-evolving landscape of chemical research.